Tim Clarey, Ph.D., and Vernon R. Cupps, Ph.D.*
 When most people think about radioisotope dating, they think of carbon-14 (C-14), or radiocarbon dating.1 However, C-14, a radioactive variety of carbon, decays too quickly to use on rocks that secular scientists think are millions of years old. With such a fast decay rate, any radiocarbon in a sample would be undetectable in less than 100,000 years.
When most people think about radioisotope dating, they think of carbon-14 (C-14), or radiocarbon dating.1 However, C-14, a radioactive variety of carbon, decays too quickly to use on rocks that secular scientists think are millions of years old. With such a fast decay rate, any radiocarbon in a sample would be undetectable in less than 100,000 years.
That’s why geologists use other radioisotope dating methods with really slow decay rates (long half-lives) to claim great ages for rocks and, hence, the earth. These include the 40K-40Ar (potassium-argon), 40Ar-39Ar (argon-argon), 87Rb-87Sr (rubidium-strontium), 147Sm-143Nd (samarium-neodymium), U-Pb (uranium-lead), and the 206Pb-207Pb (lead-lead) dating methods.
Each method makes several basic assumptions.2 First and foremost, each method assumes that the radioisotope decay rate has never changed during a rock’s entire existence. Second, each method has to assume a starting amount of both parent and daughter isotopes. Third, all methods assume that no isotopes have been washed in or out by groundwater, changing the amounts.
Although a constant decay rate might seem reasonable, ICR’s Radioisotopes and the Age of the Earth (RATE) project clearly demonstrated that the decay rates of the radioisotopes used by dating methodologies likely accelerated at some time in the past—i.e., they did not remain constant.3
The next step involves expressing the decay rate as a half-life and inserting it into the general age equation below. A half-life is the time it takes for half of the original radioactive element (parent isotope) to decay into another element (daughter isotope).
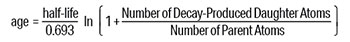
This equation implicitly assumes that the starting number of daughter atoms in the rock is known. Knowing the starting amount of daughter element and the amount of decay is critical to calculate an accurate age. Also, groundwater flowing through the rocks can change parent and daughter amounts over time. Some will dissolve, and others may be added. Yet the equation assumes this is not the case.
Secular scientists are stuck with a single equation that has multiple unknowns. And any one equation by itself can only determine one unknown. Ask your math teacher. To solve for more unknowns, you would need an equal number of equations. To fill in the blanks, secular geologists assume the original daughter amount and plug it into the equation. And because today’s half-lives are measured in millions or billions of years, the assumption of a constant decay rate virtually guarantees scientists get a great age as a result. But without a time machine, there’s no method to test if their answer is correct. This method is precise but not necessarily accurate. And radioisotope dates for rocks of known age (i.e., historical volcanic eruptions) are usually greatly in error!4
Finally, the model used for some other radioisotope dating methods—the isochron dating model—doesn’t unambiguously reproduce linear relations with age information from the raw data. In fact, the raw data appear to be better explained by isotope mixing.5,6
In summary, radioisotope dating doesn’t accurately date rocks from recent volcanic eruptions,4 and the various methods often contradict each other.3 There is strong evidence that decay rates have varied in the past. And the primary model uses isotope ratios and unfounded assumptions to derive an age.
Creation scientists are still working to answer questions related to radioactive decay.7 But given its contradictions and built-in assumptions, radioisotope dating doesn’t and can’t prove an old earth.
References
- Morris III, H. et al. 2020. Creation Basics & Beyond, 2nd ed. Dallas, TX: Institute for Creation Research, 335-342.
- Ibid, 323.
- Vardiman, L., A. A. Snelling, and E. F. Chaffin, eds. 2005. Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative. El Cajon, CA: Institute for Creation Research and Chino Valley, AZ: Creation Research Society.
- Clarey, T. 2020. Carved in Stone: Geological Evidence of the Worldwide Flood. Dallas, TX: Institute for Creation Research, 80-81.
- Cupps, V. 2020. Revisiting the Isochron Age Model, Part 1. Acts & Facts. 49 (6): 10-13.
- Cupps, V. 2020. Revisiting the Isochron Age Model, Part 2. Acts & Facts. 49 (7): 10-13.
- For example, how did God safely dissipate the heat generated by accelerated nuclear decay?
* Dr. Clarey is a current Research Associate and Dr. Cupps is a former Research Associate at the Institute for Creation Research. Dr. Clarey earned his Ph.D. in geology from Western Michigan University, and Dr. Cupps earned his Ph.D. in nuclear physics at Indiana University-Bloomington.