An Introduction to Gene Complexity
Genes are found on both strands of the double-stranded DNA molecule (Figure 1). Their complexity has many facets. First, the boundaries of what can be called a single gene and its complete set of functions are becoming increasingly hard to delineate. Entire chromosomes and genomes are a continuum of pervasive and overlapping transcription (copying DNA into RNA).2,3 Recent discoveries have revealed that the genes of many plants and animals are not like single entities at all but are a mixture of genes within genes and even genes that overlap each other (Figure 1).3 The regulatory control regions of genes, called promoters, can be shared by two completely different genes whose transcriptions run in opposite directions from each other.
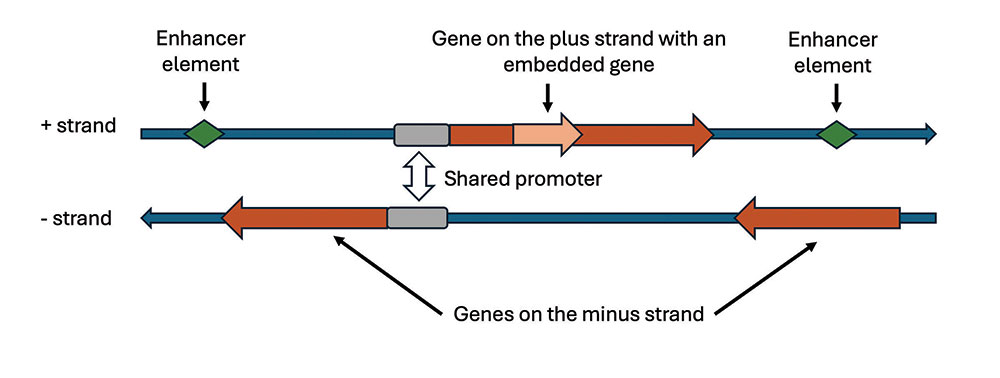
Enhancer (regulatory) elements that also play an important role in regulating gene function can be up to a million bases away from the gene they regulate. As if this weren’t enough, many genes function both forward and backward at the same time, producing both sense and antisense transcripts.4 The regulatory sequences of genes can also be located inside other nearby genes, and researchers have determined that genes dynamically interact with each other in “gene neighborhoods” much more than previously believed, to the point of blurring the boundaries between them.
Second, the informational output provided by genes can change depending on different circumstances that include cell type, tissue type, and other stimuli such as the external environment.5 In the genome, both the DNA molecule itself and the histone proteins that the DNA molecule is packaged around can be chemically altered or tagged. The study of these chemical tags is called epigenetics or chromatin remodeling.5,6 In addition to genes having overlapping boundaries and alternate functions, the information the genes provide is epigenetically altered by the cellular machinery to allow just the right output for the situational need at hand.5
When evolutionists talk about creatures sharing the same genes, they are typically referring to very small segments of the genes’ DNA. And in most cases, they specifically mean the protein-coding segments inside genes called exons—not the whole segment of DNA that is actually responsible for producing the information needed to make the correct version of the protein and RNA specific to each creature kind at the right time and in the correct amount.
But what about all the other expressed DNA sequences in the genome besides protein-coding segments? Can they be called genes, too? Amazingly, there are more than twice as many long non-coding RNA (lncRNA) genes in the human genome as there are protein-coding genes.2,3 These lncRNA genes produce long RNAs that are used either structurally or functionally in the cell for many different purposes. In fact, many of these are turning out to be molecules that control and regulate protein-coding genes.7,8
Alternative Splicing
Since the first viral gene was sequenced at the beginning of the modern genomics era about 40 years ago, the concept of what comprises a gene and how it works has changed markedly.1 In the ensuing time, entire microbial, plant, and animal genomes have been sequenced.
When research into gene function began, researchers widely assumed that a one-to-one relationship existed between genes and their RNA and protein products. However, genome sequencing projects soon revealed that the large number of RNAs and corresponding proteins being discovered were hundreds of times more numerous than the number of genes found in the DNA sequence.
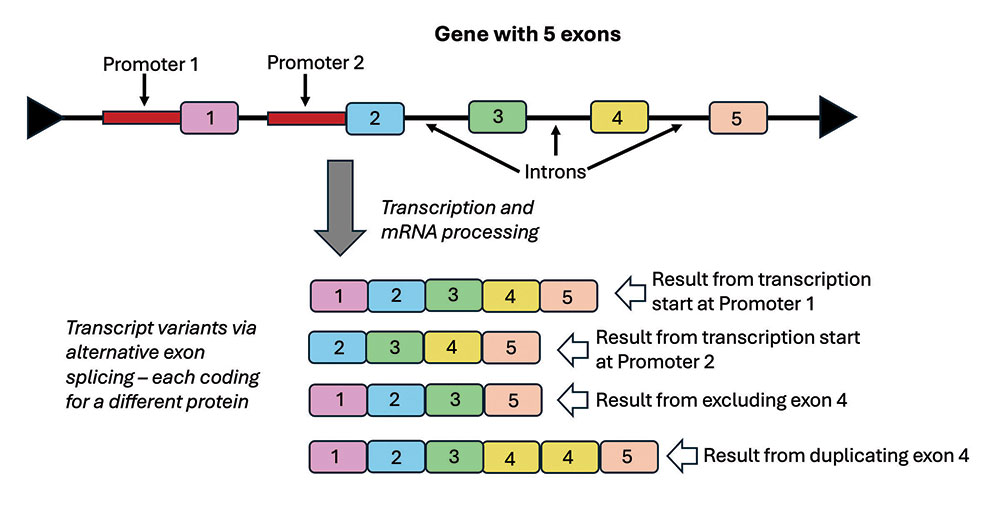
We now know that this is due to the many complex mechanisms associated with gene function. In plants and animals, a gene typically produces a messenger RNA (transcript) from multiple segments of coding DNA in a gene region (Figure 2). These coding segments are called exons, while the non-coding segments (introns) are spliced out as the RNA is processed (Figure 2). A single gene region can produce a variety of transcripts by adding, multiplying, or eliminating exons in a process called alternative splicing (Figure 2).9 For example, a mere three neurexin genes in humans can produce over 3,000 different transcripts.10
Scientists deduced a predictive model for the splicing code of many genes that has been able to predictively account for about 80% of the transcriptional output of the genes that were studied.10 This model employed a diverse set of factors and analyzed the alternative splicing across 27 different mouse tissue types.11 In brief, researchers discovered that the splicing code model involves:
1. diverse regulatory DNA sequences functioning as control features located throughout gene regions,2. complex interconnections between genes and gene networks,
3. dynamic regulation of three-dimensional chromosome architecture,
4. the interplay of DNA chemistries and conformational features,
5. cell tissue type and physiological state, and
6. the effects of DNA sequence variation within populations.10
Even these categories can be further broken down into subfields of study.
Long Complex Gene Tails
If the picture of gene regulation and splicing were not complicated enough, a very interesting study increased this paradigm to an unprecedented level.12 Performed in both mice and humans, this study showed that not only are a gene’s exons alternatively spliced but so is the sequence called the 3-prime untranslated region (3' UTR) that’s tagged on to the end of a gene like a tail.12 This 3' UTR tail does not code for proteins but instead contains a variety of genetic switches that help enable the gene to be regulated after it is transcribed.
Some of these 3' UTR gene tail features allow regulatory RNAbinding proteins to attach to the mRNA’s tail, while others allow small regulatory RNAs called microRNAs to bind.12,13 The combination of these bound regulatory molecules fine-tunes and robustly controls genes after the mRNAs are produced. This form of regulation is called post-transcriptional because it happens after the mRNA is transcribed. And like the coding areas of the gene, these 3' UTR tails are also alternatively spliced and thus variable. Their size and makeup can vary widely and dynamically between mRNAs from the same gene and between the different cell types in which they are found.
While scientists knew about the diversity of 3' UTRs gene tails before this study, they discovered that this feature was on a much more intricate and massive scale than they anticipated. In fact, they identified 2,035 mouse and 1,847 human genes that have 3' UTR tails ranging from 500 to 25,000 bases long. In some cases, they were even longer than the protein-coding areas of the genes themselves. These incredibly long gene tails literally contain hundreds to thousands of genetic switches within each single mRNA. The complexity of genetic control at this level astounds researchers.
Alternative Splicing and Adaptation: Insects
Many past studies connect gene expression levels to various types of adaptation in plants and animals in response to environmental changes. However, most looked at the quantity of mRNAs produced from certain genes and the alternative activation of different genes, not alternative splicing. In recent years, several studies done with insects and vertebrates have shown that a number of genes undergo alternative splicing in adaptation.14

One example is within insect castes, which are groups of individuals within the same kind of social insect (e.g., ants, bees, termites) that have a different bodily appearance that’s typically related to the role of the caste within the colony. For example, members of the soldier caste in a colony of army ants are larger than the others and have huge jaws. The number of individuals in each of the insect castes can vary adaptively with environmental variables such as external threats to the colony.14
Comparative analyses between insect colonies indicate that alternative splicing is a contributing factor to caste differences in termites, honeybees, and potentially ants. In the bumble bee, 40% of genes express multiple mRNA splicing isoforms, with many being caste specific.15 In nonsocial insects that don’t have castes, alternative splicing has also been found to play a role in the seasonal plasticity of wing pattern variation in the butterfly Bicyclus anynana.15 This butterfly is one of many insect kinds that adaptively varies its coloration scheme depending on the season.15
Alternative Splicing and Adaptation: Vertebrates
Reptiles are another group of creatures that adaptively interact with their environment. At temperatures below 26°C, a temperature-sensitive regulatory enzyme in turtles called a kinase modifies a group of RNA-binding proteins that regulate splicing.16 This kinase-driven modification leads to a group of RNA-binding proteins that are translocated from the cytoplasm to the cell nucleus. Here they activate a set of regulated alternative splicing events that cause the development of male turtle embryos. Above 31°C, the kinase becomes inactive, leading to alternative splicing events that promote female development. At intermediate temperatures, a mixture of both sexes will develop.

Adaptive alternative splicing has also been observed in fish. In the salmonid fish called Arctic char there exist benthic (deep-water dwelling) and pelagic (near-surface dwelling) ecotypes of the same species across multiple independent lakes.17 These two different ecotype adaptations are characterized by differences in swimming ability resulting from alternatively spliced genes that produce regulatory proteins that are top-level regulators of specific gene networks.
Similarly, researchers also documented this type of adaptive response in a cichlid fish where rapid ecological adaptation along depth gradients was discovered in Lake Masoko, an African crater lake.18 The cichlid Astatotilapia calliptera has diversified into pelagic and deep benthic ecotypes with strikingly different jaw structures. Between the two ecotypes, researchers identified 7,550 genes with alternative splicing. About 15% of those genes were associated with craniofacial development leading to different jaw forms. The researchers reported that alternative splicing played a large role in “driving ecologically relevant divergence in gene expression during adaptive diversification.”18
An example of adaptive splicing in mammals is the house mouse. In a recent study, researchers studied mice in a latitude gradient on the East Coast of the United States.19 Mice were sampled in Florida, Georgia, Virginia, New York, and New Hampshire/Vermont. As is true with Bergmann’s rule,20 in Eastern North America mice in colder environments are physically larger, build bigger nests, differ in metabolic traits, and show differences in gene expression compared to mice from warmer environments.19
In the mouse study, researchers specifically looked at differences in alternative gene splicing. The analyses identified a small set of alternatively spliced transcripts correlated with environmental adaptation. Many of these alternatively spliced genes were connected to traits associated with body size, which was observed to gradually increase with latitude. Interestingly, there was no overlap between previously identified genes that were associated with changes in mRNA abundance. These results indicate that alternative splicing and changes in mRNA abundance are separate molecular adaptation mechanisms.
Conclusion
With each passing year, research into the inner workings of the genome reveals ever-deepening levels of intricately engineered processes, especially as they relate to gene complexity. To quote Dorothy from the classic movie The Wizard of Oz, “I’ve a feeling we’re not in Kansas anymore.” In fact, the biocomplexity of the genome is now reaching proportions beyond our wildest imaginations and even our ability to understand.
Most importantly, the naïve simplicity of the Darwinian paradigm of random mutations operated on by a mystical selective agent is clearly falling apart in the face of such vast, complex, precise, and elegant bioengineering. Only our omnipotent and all-wise Creator, the Lord Jesus Christ, is capable of providing a sane and viable answer to the origin and operation of life on Earth.
References
- Portin, P. 2009. The Elusive Concept of the Gene. Hereditas. 146 (3): 112–117.
- Djebali, S. et al. 2012. Landscape of Transcription in Human Cells. Nature. 489 (7414): 101–108.
- Clark, M. B. et al. 2013. The Dark Matter Rises: The Expanding World of Regulatory RNAs. Essays in Biochemistry. 54: 1–16.
- Antisense transcripts are believed to help regulate the genes from which they are copied in reverse, possibly by binding to the sense RNA transcripts directly or by facilitating the splicing and processing of the sense transcripts. See Pelechano, V. and L. M. Steinmetz. 2013. Gene Regulation by Antisense Transcription. Nature Reviews Genetics. 14 (12): 880–893.
- Tomkins, J. P. 2023. Epigenetic Mechanisms: Adaptive Master Regulators of the Genome. Acts & Facts. 52 (4): 14–17.
- Zhu, J. et al. 2013. Genome-Wide Chromatin State Transitions Associated With Developmental and Environmental Cues. Cell. 152 (3): 642–654.
- Managadze, D. et al. 2013. The Vast, Conserved Mammalian lincRNome. PLoS Computational Biology. 9 (2): e1002917.
- Information for this section was drawn from Tomkins, J. P. 2014. Gene Complexity Eludes a Simple Definition. Acts & Facts. 43 (6): 9.
- Horiuchi, T. and T. Aigaki. 2006. Alternative Trans-Splicing: A Novel Mode of Pre-mRNA Processing. Biology of the Cell. 98 (2): 135–140.
- Reissner, C., F. Runkel, and M. Missler. 2013. Neurexins. Genome Biology. 14, article 213.
- Barash, Y. et al. 2010. Deciphering the Splicing Code. Nature. 465 (7294): 53–59.
- Miura, P. et al. 2013. Widespread and Extensive Lengthening of 3' UTRs in the Mammalian Brain. Genome Research. 23: 812–825.
- Tomkins, J. P. 2024. Small Heritable RNAs Pack a Big Adaptive Punch. Acts & Facts. 53 (1): 12–15.
- Haltenhof, T., M. Preußner, and F. Heyd. 2024. Thermoregulated Transcriptomics: The Molecular Basis and Biological Significance of Temperature-Dependent Alternative Splicing. Biochemical Journal. 481 (15): 999–1013.
- Harvey, J. A., L. S. Corley, and M. R. Strand. 2000. Competition Induces Adaptive Shifts in Caste Ratios of a Polyembryonic Wasp. Nature. 406: 183–186.
- Wright, C. J., C. W. Smith, and C. D. Jiggins. 2022. Alternative Splicing as a Source of Phenotypic Diversity. Nature Reviews Genetics. 23 (11): 697–710.
- Jacobs, A. and K. R. Elmer. 2021. Alternative Splicing and Gene Expression Play Contrasting Roles in the Parallel Phenotypic Evolution of a Salmonid Fish. Molecular Ecology. 30 (20): 4955– 4969.
- Carruthers, M. et al. 2022. Ecological Speciation Promoted by Divergent Regulation of Functional Genes Within African Cichlid Fishes. Molecular Biology and Evolution. 39 (11): msac251.
- Manahan, D. N. and M. W. Nachman. 2024. Alternative Splicing and Environmental Adaptation in Wild House Mice. Heredity. 132 (3): 133–141.
- Named after nineteenth-century German biologist Carl Bergmann, this rule holds that organisms in colder climates are usually larger than related organisms in warmer climates. See Bergmann, C. 1847. Ueber die Verhaltnisse der Warmeokonomie der Thierezuihrer Grosse. Gottinger Studien. 3: 595–708.