Our world is dynamic, offering changes and challenges to its living residents. Plant and animal trait variations can help them adapt to certain settings. Some adapt quickly as they pioneer new niches, developing traits to fit the environmental conditions. How does this happen?
Our world is dynamic, offering changes and challenges to its living residents. Plant and animal trait variations can help them adapt to certain settings. Some adapt quickly as they pioneer new niches, developing traits to fit the environmental conditions. How does this happen?
Two 19th-century pioneers investigated this question. Charles Darwin (1809–1882) observed pigeons (Columba livia).1 He noticed that certain hybrids suddenly displayed feather patterns or other traits that neither parent breed had shown. Gregor Mendel (1822–1884) saw certain traits suddenly appear in pea plants (Pisum sativum) that he studied for eight years. Where did the new variations come from? Mendel and Darwin gave different answers.
Darwin Versus Mendel in the Trait Variation Question
 Darwin recognized the role of human breeders who select individual animals for certain traits. He wrote, “The key is man’s power of accumulative selection: nature gives successive variations; man adds them up in certain directions useful to him.”2 He then imagined nature doing an even better job than humans of producing endless trait varieties.
Darwin recognized the role of human breeders who select individual animals for certain traits. He wrote, “The key is man’s power of accumulative selection: nature gives successive variations; man adds them up in certain directions useful to him.”2 He then imagined nature doing an even better job than humans of producing endless trait varieties.
But how did he determine that man’s selective power—not a creature’s innate capability—is “the key” to trait variation? He just chose to believe it.3 And thus, Darwin’s concept of natural selection became the prime mover of today’s molecules-to-man evolutionary story.
Mendel observed that flower petal colors appeared in hybrid plants that were different colors from the parent plants. He tabulated hundreds of results from specific plant traits that switched on or off. For example, wrinkly versus smooth peas in the pods occurred in 1:3 ratios in the third generation.
parent plants. He tabulated hundreds of results from specific plant traits that switched on or off. For example, wrinkly versus smooth peas in the pods occurred in 1:3 ratios in the third generation.
Mendel noted that if inherited characteristics came in two versions, which we now call alleles, they would explain the results.4 Independent sorting of alleles into sperm and egg cells would produce the ratios he tabulated.
Engineered Alleles
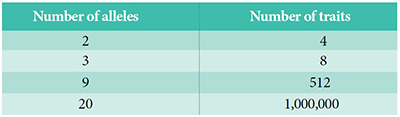
Mendel located the source of variation within, not without, living creatures. He wrote, “The number of the components, as is known, increases with the number of the differentiating characters in cubic ratio.”5 We can call this Mendel’s Law of Exponential Trait Combinations.6 All the Lord Jesus had to do to ensure enormous potential for differentiation within each created kind was to embed alleles into the first male and female of each creature.
Mendel discovered an actual key to speciation. It begins with meiosis where half of a parent’s DNA gets transferred to an egg or sperm cell. Inbred generations inherit identical alleles because they are descendants of the same set of alleles. This causes a loss of heterozygosity. Heterozygotes have more alleles to distribute to further generations than do homozygotes.
Speciation is spurred when the new combination of alleles from each parent in the offspring becomes reproductively isolated from the parents’ population. This happens in many ways, such as moving away from the parents, showing mating behaviors that differ from those of the parents, or even deploying alleles for incompatibility proteins that keep sperm from docking with eggs.
Indeed, researchers found a “deep-seated common genetic basis to reproductive isolation among very different organisms,” tomatoes and fruit flies.7 It looks like the Lord preprogrammed reproductive isolation in creatures to encourage their divergence—one way to make sure they would multiply and “fill the earth” (Genesis 1:22).
Once the new population breeds only with its own members, its particular trait combination stabilizes into a new species that now pioneers a new niche. In sum,
1. Meiosis -> Heterozygosity lost
2. Reproductive isolation -> Speciation
When formerly isolated species come back together, or hybridize, then even long-separated alleles recombine to restore heterozygosity. The offspring can immediately look and act more like the original parents.
3. Hybridization -> Heterozygosity regained
Mendel wrote, via an English translation, “Transitional forms were not observed in any experiment.”8 He saw discrete trait variations that switched off or on in predictable ratios, not traits in some succession of endless morphing. In short, he saw engineered biology.
Alleles, not Mutations
Mendel stated, “Nothing justifies the assumption that the tendency to the formation of varieties is so extraordinarily increased that the species speedily lose all stability, and their offspring diverge into an endless series of extremely variable forms.”9 In short, Mendel’s pea plants disobeyed Darwinism and stubbornly remained pea plants.
So, along came Neo-Darwinism to save the evolutionary story. This construct added mutations (Neo) to selection (Darwinism). Nature, the substitute designer in Darwin’s plan, would now select mutants as the precursors of new life forms.
But the Law of Exponential Trait Combinations has something to say about mutations. Imagine God made a creature with one billion DNA bases in its total genome.10 Let’s say He engineered only 500 alleles and that each allele occupied one DNA base—although it’s more complicated in reality, as explained below. In the beginning, God placed allelic differences at just those sites that would generate adaptive or ornamental, not fundamental, trait differences. The potential for trait variations or phenotypes in eventual offspring from 500 alleles becomes practically limitless…with zero random mutations needed!
Do random mistakes occur? Of course. Mistakes happen in today’s sin-cursed universe.11 New research even suggests that creatures corral other genetic changes into certain genetic zones, suggesting that the Divine Engineer accounted for even them. However, if created alleles are a means of adaptation, random mutations are not necessary for variation.
Mendel wrote that “the species possesses the capacity of fitting itself to its new environment.”12 Does modern research still side with such internalism?
Alleles in Action
Adaptive radiations (ARs) fascinate biologists. These occur when a founder population13 quickly diversifies into species (variously called morphs or subspecies) that inhabit new niches. Two AR examples show that encoded genetic information, not random mutation or selection, drives speciation.
A stunning array of cichlid fishes have diversified in African lakes. The three largest lakes (Malawi, Tanganyika, and Victoria) contain in the same water cichlids that are well-adapted to eating plankton, others that scrape algae off rocks, some that are suited to crush snails for food, some that nibble other fishes’ scales, and still others with big lips that eat insects.
Neo-Darwinism has each of these forms emerging through slowly accumulated random mutations. Accordingly, cichlids should have evolved those morphs first, each of which then colonized the lakes. Thus, the insect-eaters from all three lakes should have more similar genetics to one another than to the other cichlids in that lake. But recent studies have shown the opposite.
It turns out that even closely related cichlids already have many differences across their genomes. They retain “duplicate genes” that “exhibit new expression patterns.” Some of the genes code for micro-RNAs that “stabilize and refine expression patterns.”14 Inbuilt mechanisms of diversification also include transposable element insertions15 and the recruitment of “old alleles from standing variation.”16 Thus, not only does it appear that the Lord Jesus front-loaded these fish with alleles to tweak traits each time a founder population pioneers a new lake, but some alleles even stabilize those traits into new species. No random mutation or selection is needed—just engineered cichlid genomes.
 Darwin’s famous finches, Geospiza fortis, offer another example. These birds had branched into a dozen species by the time Darwin visited them on the Galapagos Islands in 1835. Geospiza species, now reclassified as tanagers instead of finches, can differ in plumage and beak shape across the Galapagos Islands that they inhabit.
Darwin’s famous finches, Geospiza fortis, offer another example. These birds had branched into a dozen species by the time Darwin visited them on the Galapagos Islands in 1835. Geospiza species, now reclassified as tanagers instead of finches, can differ in plumage and beak shape across the Galapagos Islands that they inhabit.
Do their genetics point to the selection of random mutations as the way these birds diversified? Not according to genome analyses. Geospiza genome sequencing found that “extensive sharing of genetic variation among populations was evident, particularly among ground and tree finches, with almost no fixed differences between species in each group.”17 Where did this genetic variation come from?
Researchers screened genomes of representative Geospiza birds with both blunt and pointed beaks. They concluded that “hybridization…has influenced the evolution of a key phenotypic trait: beak shape.”17 The team identified two distinct variants of a gene named ALX1 that helps control beak shape.
One year later, another team sequenced DNA from six Geospiza species to learn that another gene region called HMGA2 controls beak size.18 Evolutionary biologist Dolph Schluter told Nature News, “We can point to a physical, material basis for that change.”19 Quite unlike mystical Neo-Darwinian concepts, the material basis for trait adjustment was already built into these birds, just as it was in Mendel’s peas.
Time after time, researchers look for accumulated random mutations that they’ll expect, but they keep finding instead that preexisting alleles play the prominent role in generating variations. When blind Mexican tetra (Astyanax mexicanus) crossbreed with sighted individuals, a range of eye sizes suddenly appears. A tribe of plants called silverswords greatly diversified as they colonized Hawaii. Some grow tall with woody trunks, while others have fleshy stems that stay near the ground, yet they all hybridize.
And remember those pigeons? Recent results showed that sorting alleles drives their plumage diversity.20 Darwin was wrong, after all.
Engineered Biology
One recent report reviewed ARs. Its authors wrote:
It is well possible that increased efforts along this line of investigation will still fail to uncover general genomic features, and that the determinants of organismal diversification need to be explored otherwise.21
Where else should they look if not to “general genomic features” like mutations? Perhaps they should further explore their observation that “the genomes of these species contain adaptive allelic variants that originated long before the actual species or populations have formed.”22 How long before? Try the beginning of creation.
What has the last century revealed about adaptations? First, random mutations do very little for adaptation, and none are needed. Second, alleles most often consist of networks made of genes, regulatory elements, and other linked features. We have also learned that many adaptations arise from mixing and matching alleles already within creatures, not from factors outside of them.
To Biology and Beyond!
Those who regularly read Acts & Facts should be familiar with continuous environmental tracking (CET), ICR’s engineering-based biological model of adaptation.23 CET discussions have pointed to an array of adaptive mechanisms—including transposable elements, mutational hotspots, post-transcriptional editing, epigenetic mechanisms, etc.—that creatures use to track their surroundings, process those data inputs, and deploy suitable trait adjustments even in later generations.
How does this fit with the conclusion that “rapidly- and extensively-diversifying lineages seem to be those having access to a pool of alleles useful for the adaptation to novel ecological niches”?21 We suggest that the Lord integrated this “pool of alleles” with CET-related mechanisms in each of His creatures.
For that matter, a creature’s environmental tracking processes could influence allele sorting during meiosis—all by design. We already see evidence of “composite elements combining multiple coding and regulatory variants at several individual genes” and that these reveal a “daunting complexity underlying adaptive divergence.”21
Clues that suggest creatures were engineered to adapt include the repeatability of trait deployment, suitability of trait variations to specific ecologies, the rapidity of trait adjustments, predictability of traits based on allele sorting, and a pool of alleles for adaptive or ornamental rather than fundamental traits. The Lord built a brilliant system that maximizes potential for phenotypic diversity while minimizing genetic storage space. Zero random mutations and external selections are required to generate plenty of variants from each created kind.
It appears that our Lord wanted variety in His creatures, so He gave them diversity generators from the beginning.
Reference
- Darwin, C. 1869. On the Origin of Species. London: John Murray, 25.
- Ibid, 30.
- Where did Darwin derive this belief from if not from science? One evolutionist critic suggested that “Darwin needed a mechanical approximation of intelligent regulation to answer the Protestant theologians (especially Paley). He got it from the free market mechanism of Smith [via Thomas Malthus].” Brady, R. H. 1982. Dogma and Doubt. Biological Journal of the Linnean Society. 17: 79–96.
- Bateson, W. and G. Mendel. 2009, first printed 1902. Mendel’s Principles of Heredity: A Defence, with a Translation of Mendel’s Original Papers on Hybridisation. Cambridge, UK: Cambridge University Press, 67.
- Ibid, 85.
- Professor Nigel Crompton is to be credited for this wording.
- Moyle, L. In Milton, J. Animal and plant genes hard-wired for speciation. Nature News. Posted on nature.com September 16, 2010.
- Bateson and Mendel, Mendel’s Principles of Heredity, 51. Emphasis in original.
- Ibid, 82.
- Humans have about 1 billion bases, chimps 1.2 billion, and pea plants 4.45 billion.
- See Romans 8:20–22.
- Bateson and Mendel, Mendel’s Principles of Heredity, 82.
- This refers to individuals that pioneer new areas and then diversify to fill its various niches.
- Jiggins, C. D. 2014. Radiating genomes. Nature. 513 (7518): 318–319.
- See Tomkins, J. 2023. Transposable Elements: Genomic Parasites or Engineered Design? Acts & Facts. 52 (5): 14–17.
- Brawand, D. et al. 2014. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 513 (7518): 375–381.
- Lamichhaney, S. et al. 2015. Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature. 518 (7539): 371–375.
- Lamichhaney, S. et al. 2016. A beak size locus in Darwin’s finches facilitated character displacement during a drought. Science. 352 (6284): 470–474.
- Rogers, N. Evolution of Darwin’s finches tracked at genetic level. Nature News. Posted on nature.com April 21, 2016.
- Specifically, they found that this allele is a gene regulatory element. They also found that a disease- associated mutation contributes to a feather color pattern. Vickrey, A. I. et al. 2018. Introgression of regulatory alleles and a missense coding mutation drive plumage pattern diversity in the rock pigeon. eLife. 7: e34803.
- Berner, D. and W. Salzburger. 2015. The genomics of organismal diversification illuminated by adaptive radiations. Trends in Genetics. 31 (9): 491–499.
- Ibid, emphasis added.
- For more information, visit ICR.org/CET.
* Dr. Thomas is Research Scientist at the Institute for Creation Research and earned his Ph.D. in paleobiochemistry from the University of Liverpool.