Pseudogenes Are Not Pseudo Anymore
by Jeffrey P. Tomkins, Ph.D. | Oct. 31, 2025
Introduction
One of the past arguments for evidence of biological evolution in the genome has been the concept of pseudogenes. These DNA sequences were once thought to be the defunct remnants of genes, representing nothing but mutated genomic “fossils” in the genomes of plants, animals, and humans. However, much research has been published over the past several decades showing that pseudogenes are not only functional but key to organism survival.1,2
Types of Pseudogenes
Pseudogenes come in three different forms depending on their DNA sequence characteristics. The group of pseudogenes that has the largest representation in the human genome is what is called a processed pseudogene (also called a retrogene).2 There are about 10,000 of these in humans, and this category is also the most highly studied for functionality.
It has been postulated by evolutionists that these processed pseudogenes were derived from an mRNA copy of a protein-coding gene that had its intervening sequences (introns) spliced out, which were then retro-transcribed into DNA and inserted back into the genome—hence the moniker “processed.” Conventional scientists believe that this is why these types of pseudogenes lack introns and, in most cases, a promoter region similar to that of its protein-coding counterpart.3 In addition, a processed pseudogene is almost always located on a different chromosome than its alleged parent gene. Despite the seemingly accidental structure of these genes, many of them are being shown to have important functionality, including some with introns that also exhibit functionality.4
A second category of pseudogenes are duplicated pseudogenes.2 These are believed to have been duplicated from a parent gene since they contain promoters and introns but have alterations in their DNA code that keep them from producing a protein. Conventional scientists believe that these may have been derived during DNA replication due to an unequal crossing-over event during meiosis.5 However, this is pure speculation just as in the case of processed pseudogenes. In fact, this hypothetical explanation is even employed for the development of large gene families that do not include pseudogenes.
The third category of pseudogenes is rare in the genome and called a unitary pseudogene.2 These don’t necessarily have an obvious parent from which they are derived. Although, if they are part of a gene family, duplication will often be invoked as an evolutionary meme. These types of genes will often have DNA code alterations that prohibit a protein from being produced and are in most cases considered to be broken genes. However, the human beta-globin pseudogene, which was once considered to be a broken gene, has actually now been shown to be highly functional by producing long non-coding RNAs (lncRNAs) that help regulate the entire beta-globin gene network.6,7 Furthermore, this gene is highly intolerant of mutations, which can cause beta-thalassemia disease pathologies.5
Functional Roles of Pseudogenes
Despite the still-prevalent myth that pseudogenes are genomic fossils, scientists have been identifying important functions for them in mammals since 1985.8,9 A case in point is the discovery of life-critical function for the human retrogene PPM1K.10 Scientists first realized that the PPM1K pseudogene was being actively transcribed. When the cells of cancer patients were examined, the regulatory lncRNAs produced by PPM1K were found in abnormally low levels compared to healthy humans. The PPM1K pseudogene RNAs were not only found to help regulate the protein-coding version of the PPM1K gene, from which it supposedly evolved, but also the NEK8 gene that has also been associated with cancerous cell growth. Therefore, if this pseudogene is not functioning properly, cellular dysfunction and cancer are the likely result.
Pseudogenes work their functional expertise through a variety of molecular mechanisms and systems. In one of the leading systems, pseudogenes are thought to participate as biological regulators by functioning as competitive endogenous RNAs (ceRNAs). In a previous article, I showed that small RNAs called microRNAs (miRNAs) are produced from RNA copies of long DNA sequences (long primary miRNAs) and then are eventually processed into smaller mature miRNAs that are only about 19 to 25 nucleotides long.11 These miRNAs then bind to other RNA transcripts such as lncRNAs, protein-coding gene transcripts, and pseudogene transcripts at specific target sites called miRNA response elements (MREs). This competitive binding among populations of RNA transcripts and miRNAs is a complicated cellular system of titrating and fine-tuning gene regulation (Figure 1).2,12
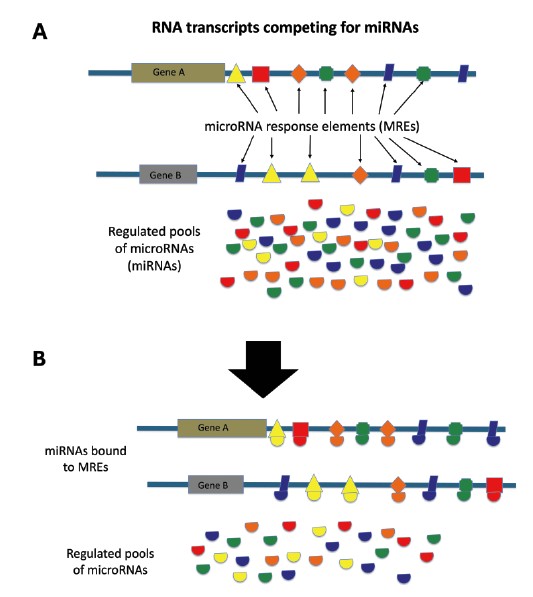
One of the most widely studied pseudogenes in humans is PTENP1, which is an unprocessed pseudogene similar in sequence to its alleged parent protein-coding gene PTEN.2,13 The ceRNAs produced from the PTENP1 pseudogene along with a host of other important genes control cell growth. When PTENP1 is disrupted, cancerous growth is often the result. The lncRNA-ceRNA encoded by PTENP1 helps control the concentration of PTEN-regulating miRNAs by acting like a sponge and maintaining their proper levels in the cell.2 But the complexity of PTENP1 is much deeper than this since it also produces several other types of regulatory RNA molecules in the other direction on the opposite DNA strand, creating what are called antisense transcripts.14 And these RNA products play completely different roles in how they regulate the PTEN gene. Not only does this amazing pseudogene code for functional RNA molecules in both forward and reverse directions, but it also does so from multiple start sites so that it can produce different functional variants of the RNAs. And the RNA products that it produces regulate other genes, which interact with a variety of proteins and other RNA molecules in a complex regulatory network that is only just beginning to be understood.
Another role of pseudogene function involves interactions between their lncRNAs and proteins.2 Part of the cell’s biology includes specialized proteins that bind to RNAs called RNA-binding proteins or RBPs. These RBPs function as key regulators of a variety of posttranscriptional processes, including RNA splicing, transcript stability (protection from degradation), RNA transport to specific cellular addresses, and making proteins from the transcript, which functions as a template (translation). And just as miRNAs competitively function between pseudogenes and their protein-coding counterparts, so do RBPs, which bind to specific RNA-binding domain (RBD) sites on the RNAs. Several studies in humans have shown that proper functioning of pseudogene transcripts regulated by RBPs is key to maintaining healthy insulin levels and keeping cancer at bay.2
Pseudogene transcripts have also been shown to be key regulating factors in epigenetics—a topic I discussed in a previous article.15 In this respect, both the sense and antisense transcripts of pseudogenes can bind to specific chromosomal locations and regulate the transcription of their protein-coding counterparts.2 They can also bind to the transcripts of their protein-coding counterpart and regulate post-transcriptional events.
Yet another fascinating aspect of some pseudogenes is their ability to harbor what are known as small interfering RNAs (siRNA), which are sequences that are processed out of the pseudogene transcripts.2 I discussed the role of siRNAs in a previous article.11 These siRNAs can regulate the pseudogene’s protein-coding counterparts by forming specific stem-loop structures that bind to corresponding sites on the transcripts of protein-coding genes, which can repress their activity as another means of gene regulation.
Finally, more and more pseudogenes are being discovered that actually code for proteins and small proteins called peptides.2 Many of these have been discovered in the process of studying protein-coding genes that share sequence similarity to pseudogenes involved in cancer research.
Pseudogenes in Adaptation

The Mexican cavefish (Astyanax mexicanus) is a tetra that is known for its radical adaptations to cave conditions, which involve eye loss along with anatomical, physiological, and behavioral compensations.16 While the adaptive mechanisms in this fish have been heavily studied, only recently have pseudogenes been analyzed in the genomes of cavefish occupying different cave systems.17 In a 2024 study, the researchers sequenced the genomes from cavefish occupying a diversity of cave systems and found that there was a “distribution of cave-specific pseudogenes,” implying that these fish were deploying specific variants of pseudogenes that were adaptive to the cave conditions in which they were living.17 Cave conditions can vary in pH, conductivity, oxygen levels, chemistry, and the diversity of fauna that are present.

The Antarctic icefish comprises 16 different species in the suborder Notothenioidei (family Channichthyidae) and is the only vertebrate creature known to lack red blood cells. It relies instead upon diffusion-based transport of dissolved oxygen in its clear-looking blood. These unusual fish also are unique in that they can live in near-freezing ocean water around the Antarctic. One of the ways they adapt to the cold is by producing antifreeze glycoproteins (AFGPs) that are encoded by a set of AFGP protein-coding genes and accompanying pseudogenes at a specific chromosomal location. A recent study has shown that based upon the location in the ocean around Antarctica, which varies in water temperature according to latitude, the number of AFGP genes and pseudogenes will also vary.18 In fact, icefish that live at higher latitudes in warmer water will have more AFGP pseudogenes than those living in colder water.
As I discussed above, one of the main functions of pseudogenes is producing RNAs that bind to the same miRNAs as their protein-coding counterpart’s transcript in ceRNA regulatory systems. This design feature is likely also involved in fine-tuning environmentally adaptive gene expression. And just like the cavefish, adaptive pseudogene content in the icefish appears to be environment-specific.
Conclusion
It is nearly impossible to imagine how pseudogene systems this complex could arise by naturalistic processes. And certainly, the term “pseudo” now appears to be a highly inaccurate description of these misunderstood types of genes that are now believed to be almost as plentiful in number as protein-coding genes in the genome. In reality, they are just another class of lncRNA genes. The only logical conclusion is that divine engineering by an omnipotent and wise creator is the source for this amazing biological and purposeful code.
So why do evolutionary predictions about the genome continually fail in the light of new research? It’s because scientists who possess an evolutionary mindset are always viewing the genome as the product of chance or random processes. Instead, we should be looking at the genome from the perspective of pervasive functionality and as the product of incredible bioengineering by our great Creator—the Lord Jesus Christ.
References
- Wen, Y. Z. et al. 2012. Pseudogenes Are Not Pseudo Any More. RNA Biology. 9 (1): 27–32.
- Salmena, L. 2021. Pseudogenes: Four Decades of Discovery. Methods in Molecular Biology. 2324: 3–18.
- For an explanation of how genes are structured and how they function, see Tomkins, J. P. 2025. Gene Complexity Showcases Engineered Versatility. Acts & Facts. 54 (1): 14–17.
- Fablet, M. et al. 2009. Evolutionary Origin and Functions of Retrogene Introns. Molecular Biology and Evolution. 26 (9): 2147–2156.
- For an explanation of how recombination works, see Tomkins, J. P. 2024. Genetic Recombination: A Regulated and Designed Chromosomal System. Acts & Facts. 53 (4): 16–19.
- Tomkins, J. 2013. The Human Beta-Globin Pseudogene Is Non-Variable and Functional. Answers Research Journal. 6: 293–301.
- For an explanation of how long non-coding RNA genes are structured and how they function, see Tomkins, J. P. 2025. Long Non-Coding RNAs: The Unsung Heroes of the Genome. Acts & Facts. 54 (4): 14–17.
- Soares, M. B. et al. 1985. RNA-Mediated Gene Duplication: The Rat Preproinsulin I Gene Is a Functional Retroposon. Molecular and Cellular Biology. 5 (8): 2090–2103.
- Ciomborowska, J. et al. 2013. “Orphan” Retrogenes in the Human Genome. Molecular Biology Evolution. 30 (2): 384–396.
- Chan, W. L. et al. 2013. Transcribed Pseudogene ψPPM1K Generates Endogenous siRNA to Suppress Oncogenic Cell Growth in Hepatocellular Carcinoma. Nucleic Acids Research. 41 (6): 3734–3747.
- Tomkins, J. P. 2024. Small Heritable RNAs Pack a Big Adaptive Punch. Acts & Facts. 53 (1): 12–15.
- Karreth, F. A. et al. 2021. Pseudogenes as Competitive Endogenous RNAs: Target Prediction and Validation. Methods in Molecular Biology. 2324: 115–127.
- Johnsson, P. et al. 2013. A Pseudogene Long-Noncoding-RNA Network Regulates PTEN Transcription and Translation in Human Cells. Nature Structural & Molecular Biology. 20 (4): 440–446.
- DNA is a double-stranded molecule with genes on both strands running in opposite directions from each other.
- Tomkins, J. P. 2023. Epigenetic Mechanisms: Adaptive Master Regulators of the Genome. Acts & Facts. 52 (7): 14–17.
- Tomkins, J. P., S. Arledge, and R. J. Guliuzza. 2022. Catching the Vision: Blind Cave Fish (Astyanax Mexicanus) as a Model System for Continuous Environmental Tracking and Adaptive Engineering. Creation Research Society Quarterly. 58 (4): 289–296.
- Policarpo, M. et al. 2024. The Nature and Distribution of Putative Non-Functional Alleles Suggest Only Two Independent Events at the Origins of Astyanax mexicanus Cavefish Populations. BMC Ecology and Evolution. 24, article 41.
- Rivera-Colon, A.G. et al. 2023. Genomics of Secondarily Temperate Adaptation in the Only Non-Antarctic Icefish. Molecular Biology and Evolution. 40 (3).
* Dr. Tomkins is a research scientist at the Institute for Creation Research and earned his Ph.D. in genetics from Clemson University.
Long Non-Coding RNAs: The Unsung Heroes of the Genome
 Evolutionary theory holds that all living things came about through random, natural processes. So conventional scientists believe the genome has developed through these means, and large sections of it have therefore been assumed to be nonfunctional. These alleged nonfunctional regions supposedly are a source of new gene evolution. But these evolutionary presuppositions of nonfunctionality are being challenged by an unexpected group—members of ...More...
Evolutionary theory holds that all living things came about through random, natural processes. So conventional scientists believe the genome has developed through these means, and large sections of it have therefore been assumed to be nonfunctional. These alleged nonfunctional regions supposedly are a source of new gene evolution. But these evolutionary presuppositions of nonfunctionality are being challenged by an unexpected group—members of ...More...
Genomic Tandem Repeats: Where Repetition Is Purposely Adaptive
 Tandem repeats (TRs) are short sequences of DNA repeated over and over again like the DNA letter sequence TACTACTAC, which is a repetition of TAC three times (Figure 1). In the early days of genomics, these tandem repeats were originally designated as nonfunctional or junk DNA. With the exception of a few cases of tandem repeats being involved in human disease, this type of repeat variation was often believed to be neutral in its effect on the ...More...
Tandem repeats (TRs) are short sequences of DNA repeated over and over again like the DNA letter sequence TACTACTAC, which is a repetition of TAC three times (Figure 1). In the early days of genomics, these tandem repeats were originally designated as nonfunctional or junk DNA. With the exception of a few cases of tandem repeats being involved in human disease, this type of repeat variation was often believed to be neutral in its effect on the ...More...
The 3-D Genome: A Marvel of Adaptive Engineering

In eukaryotes, which are organisms with nucleated cells, the vast majority of hereditary and coded information is stored, copied, and replicated in the chromosomes within the nucleus.1 In human beings, the genetic (chromosomal) material within a single cell is about six feet in length. This needs to fit within an incredibly small space, so our Creator designed an elaborate packaging system to organize and compact ...More...
Gene Complexity Showcases Engineered Versatility
More...
More Articles
- RNA Hoops: When Circular Reasoning Makes Sense
- Engineered Parallel Gene Codes Defy Evolution
- Genetic Recombination: A Regulated and Designed Chromosomal System
- Galápagos Finches: A Case Study in Evolution or Adaptive Engineering?
- RNA Editing: Adaptive Genome Modification on the Fly
- Small Heritable RNAs Pack a Big Adaptive Punch
- Trait Variation: Engineered Alleles, Yes! Random Mutations, No!
- Transposable Elements: Genomic Parasites or Engineered Design?
- Epigenetic Mechanisms: Adaptive Master Regulators of the Genome
- Jupiter's Young Moons
- The Final World: Renovation or New Creation?
- Creation Ex Nihilo Through Jesus Christ
- James Webb Telescope vs. the Big Bang
- Evolutionary Dinosaur Myths Debunked







