 The hypothetical naturalistic origin of life and its most basic biomolecules from non-living matter is called abiogenesis. This paradigm lies at the very foundation of biological evolution, but the immensity of its naturalistic improbability is often brushed aside by evolutionists, who prefer to focus on other facets of evolution that seem less daunting.
The hypothetical naturalistic origin of life and its most basic biomolecules from non-living matter is called abiogenesis. This paradigm lies at the very foundation of biological evolution, but the immensity of its naturalistic improbability is often brushed aside by evolutionists, who prefer to focus on other facets of evolution that seem less daunting.
While virtually all of the evolutionary story lacks empirical support, its greatest impediment is the fundamental problem of how life first arose. ![]()
While virtually all of the evolutionary story lacks empirical support, its greatest impediment is the fundamental problem of how life first arose. Ultimately, life requires DNA, RNA, and protein in a system in which each molecule is dependent on the other two to both exist and function in the cell. It’s even more confounding than a chicken-and-egg scenario as to which came first. Furthermore, since each type of molecule carries and conveys complex encoded information, an intelligent information originator is the only logical cause. Code implies a coder, not naturalistic random processes.
Early Earth Atmosphere Conundrum
When evaluating the plausibility of abiogenesis scenarios, it’s critical to carefully consider the substances that would be needed for the formation of the first biomolecules: purines, pyrimidines, amino acids, sugars, and lipids. In living cells, these essential building blocks are used to form the large biomolecules and cellular structures of life. All biological molecular activities occur inside the confines of a cell, which protects them from degradation by the environment. At present, the earth’s atmosphere is about 21% oxygen by volume. The presence of atmospheric oxygen leads to oxidation, a process destructive to biomolecules that are not safely contained inside a cell. The current mass of air surrounding the earth is called an oxidizing atmosphere and is not conducive to abiogenesis.
Because oxidizing conditions rapidly destroy DNA, RNA, proteins, and cell membranes, evolutionists proposed that Earth’s early environment had a reducing atmosphere with very little or zero oxygen. They also speculate that the air must have been rich in nitrogen, hydrogen, and carbon monoxide in order to assist the spontaneous appearance of basic life building blocks such as amino acids, sugars, and nucleotides.
One big problem with this whole imaginary scenario is that geological data clearly indicate the earth’s atmosphere has always been similar to its current oxidizing conditions. Earth has always had significant levels of oxygen in its atmosphere, thus thwarting any idea of a reducing atmosphere favorable to abiogenesis.1-6
To get around the atmospheric problem, researchers have proposed that isolated localized reducing environments could have existed around volcanic plumes.7 However, these environments have extremely high temperatures and strong acidity and would simply not allow the formation of biomolecules. Some evolutionists might try to claim that “extremophile” microorganisms live in these environments today, but these creatures contain highly specialized and complex cellular systems that allow this—there is nothing primitive about them. Therefore, they are not representative of an ancient simple cell prototype (or proto-cell) that evolutionists believe might have existed.
David Deamer, a leading abiogenesis researcher, clearly stated the current status of this highly significant but largely unpublicized origins problem:
Someone from the outside world would be astonished by the lack of agreement among experts on plausible sites [for abiogenesis], which range all the way from vast sheets of ice occasionally melted by giant impacts, to “warm little ponds” first suggested by Charles Darwin, to hydrothermal vents in the deep ocean, and even to a kind of hot, mineral mud deep in Earth’s crust.8
Missing Ingredients for the First Biomolecules
Before the evolutionary process of building the first cell could begin, a diverse array of key molecules would have to be magically generated in some primordial matrix or soup. Such molecules would have to include various amino acids, pyrimidines, purines, lipids, and sugars. The alleged spontaneous generation of these key building blocks in the proper forms and amounts is an impossible hurdle. Putting these basic molecules together via naturalistic processes into larger chains and structures containing the vital molecular information needed for life is also impossible!
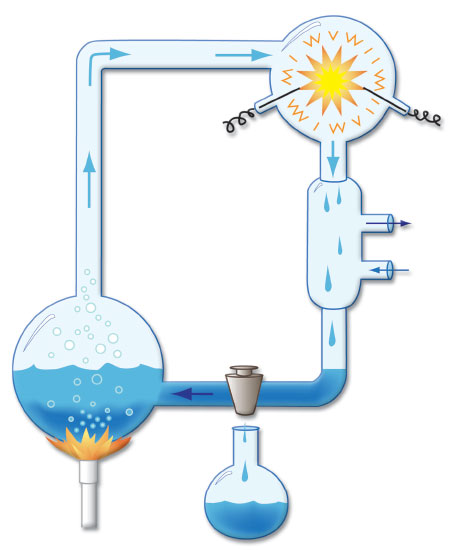
In 1952, Stanley Miller performed one of the first experiments claiming to show that naturalistic processes could produce basic molecules needed for life. Chemical gases (methane, ammonia, hydrogen, and water vapor) supposedly representing those present in an early Earth atmosphere were passed through an elaborate glass tube apparatus and blasted with an electrical discharge (Figure 1).9 Because the overall process destroyed the end products as fast as they were made, a trap was devised to capture the reactants. The toxic extract that resulted from this process was then analyzed for possible basic biomolecules, and trace amounts of three amino acids used in proteins were identified. A reanalysis of the archived samples in 2008 using more sensitive detection equipment identified three more amino acids used in proteins, but at very low levels.10
In the most successful variations of these experiments, scientists have been able to produce only 10 of the 20 amino acids used in biological proteins. However, there are very serious problems even with these meager results. First, the amino acids produced in these studies were only the most simple. Many of the amino acids used to make proteins are more complex and cannot be created using these techniques. They have elaborate side chains or molecular groups that can only be produced with complicated biochemical pathways in living cells. Second, amino acids can exist in both right- and left-handed versions, just like human hands. Miller-like experiments produce both types, but living cells can only use left-handed amino acids—not a mixture. Third, the trivial amount of amino acids the experiments produced were far too diluted to be of any biological use.
The Polymerization Problem
Another impossible hurdle for abiogenesis is the chaining together (polymerization) of base molecules like amino acids and nucleotides to form proteins and DNA/RNA. In living cells, chemical bonds link these molecules together. They are assembled according to the cell’s genetic information in a highly directed process that uses specific forms of molecular energy and complex enzyme (protein) machinery in sequences that are specifically ordered.
In 1958, Sydney Fox published a set of experiments using solutions of pure left-handed amino acids that bore no resemblance to the products of Miller’s experiments. Fox baked the mixtures, driving out the water and linking the amino acids together in crude aggregates.11 He observed the clustering of these crude spheres when placed in certain solutions and claimed these were not only a primitive form of polymerization but the beginning of cells! Of course, scientists now agree this research provided no evidence of either.
In 2004, scientists reported that carbonyl sulfide oxide, a simple chemical in volcanic gases, can activate amino acids to form peptide bonds.12 However, no more than a few amino acids could be connected, and chains of any significant size could not be produced. At present, evolutionists still have no plausible mechanism for the polymerization of proteins or nucleic acids under primitive naturalistic conditions.
An Imaginary RNA World
Biophysicist Alex Rich proposed in 1962 that RNA might have been the first to evolve.13 Researchers thought RNA could provide both informational and enzymatic qualities in the beginning, thus bypassing the necessity of DNA and proteins. The idea that RNA was the first biomolecule slowly gained traction, becoming very popular in the 1980s when research showed that specific types of RNA were involved in enzyme-like behavior in the complex processing of RNA messages copied from genes in eukaryotes. Scott Gilbert, one of the main scientists involved in these discoveries and the promotion of RNA as the original biomolecule, coined the term “RNA world.”14
But RNA world theorists generally avoid the chief problem of where the original building blocks could have possibly come from to even make RNA. Nucleic acids like RNA are complex chains of nucleotide subunits. The nucleotides themselves are also composed of a diversity of distinct molecular subunits that contain one of four different nucleobases, a ribose sugar, and a phosphate group. The nucleobases of RNA can be two different purines (adenine and guanine) or two different pyrimidines (cytosine and uracil). Before evolutionists can even consider whether RNA was the first biomolecule, they must explain the spontaneous naturalistic origin of not only the nucleotides themselves but also their molecular subunits. Another complete impediment to evolution is the overwhelming impossibility of naturalistic processes spontaneously producing these molecules outside the complex biochemistry of a fully functioning cell.
But let’s assume that all four RNA nucleotides somehow magically appeared in just the right quantities and with just the right primordial soup chemistry. The question then arises as to what chained them together to form an RNA molecule. Just as with the formation of proteins from amino acids, we run into the recurring polymerization problem. Like proteins, the formation of RNA (polymers of nucleotides) also requires complex cellular machinery. It simply can’t occur spontaneously.
But let’s give the evolutionist even more imaginary slack and suppose that RNA molecules with meaningful biological information inexplicably burst onto the scene in a chemical milieu favorable to both RNA stability and life. Yet another key obstacle to the RNA world is how the first RNA replicated itself. After all, in order for this magical RNA to continue its extraordinary journey along the eons of evolution, it must be able to perpetuate.
In 2016, researchers claimed to provide a solution. They allegedly showed that RNA could be partially replicated without lengthy protein enzymes.15 But they actually cheated because small chains of amino acids called peptides were utilized to keep the replicated products of the short RNAs from binding to each other. Peptides are shorter versions of proteins, so the RNA replication process was not really solely RNA-based, and it was also not very efficient or reliable. And the study contained some major scientific errors and could not be reproduced. As a result, the research had to be retracted, after which the authors stated, “In retrospect, we were totally blinded by our belief…we were not as careful or rigorous as we should have been.”16
Another critical issue negating the RNA world scenario is that even if RNA molecules with meaningful biological information somehow miraculously appear, RNAs are inherently unstable and quickly degrade outside a cellular environment. It goes without saying that such RNA-favorable conditions would not have been a part of the primordial soup on an early earth.
“Primitive” Lipids and Cell Membranes?
Complex biomolecules are quickly destroyed outside the protective enclosure of a living cell. Thus, some evolutionists have proposed that the chemical reactions leading to life’s origins must have occurred within a protected and enclosed space.8 Toward this end, they speculate that primitive lipids somehow self-assembled into small sphere-like structures that then in some way captured and contained the first biomolecules. This mythical and improbable scenario supposedly led to some sort of rudimentary replicating proto-cell capable of evolving into the diversity of life we see today.
The lipids found in living cells, however, are complex structures composed of different lipid chains configured with a phosphate to form specific polar structures called phospholipids. They are very different from the simple single-lipid chains evolutionists propose were in the first proto-cell. Cell membranes are also much more than just lipids. They contain complex populations of a wide variety of imbedded proteins and carbohydrate molecules that are involved in environmental sensing, signaling, and the import and export of a wide diversity of molecules required for function and metabolism. Early cell evolution proponents encounter numerous insurmountable problems of the complexity needed for the most “primitive” of cell membranes.
Summary
In response to these glaring and obvious issues, some evolutionists have proposed that precursor molecules required for cell life came from extraterrestrial sources such as some type of planetary meteoritic bombardment or even the alleged “seeding” of Earth by space aliens! However, even these creative imaginations only push back the overall problem to how those things originated.
Only an all-wise and omnipotent Creator could have been responsible for the miracle of life’s origins and the diversity and complexity of its amazing systems. ![]()
Instead of coming to grips with the impossibility of life’s most basic molecules arising through naturalistic processes, evolutionists have focused on other “downstream” parts of the problem. But these lines of research are filled with insurmountable hurdles, too. At every level, the probability that life began through naturalistic processes is essentially zilch. Only an all-wise and omnipotent Creator could have been responsible for the miracle of life’s origins and the diversity and complexity of its amazing systems. Outside of special creation as recorded in the Bible, life doesn’t stand a chance.
References
- Zahnle, K., L. Schaefer, and B. Fegley. 2010. Earth’s Earliest Atmospheres. Cold Spring Harbor Perspectives in Biology. 2 (10): a004895.
- Davidson, C. F. 1965. Geochemical Aspects of Atmospheric Evolution. Proceedings of the National Academy of Sciences. 53 (6): 1194-1205.
- Austin, S. A. 1982. Did the Early Earth Have a Reducing Atmosphere? Acts & Facts. 11 (7).
- Abelson, P. H. 1966. Chemical Events on the Primitive Earth. Proceedings of the National Academy of Sciences. 55 (6): 1365-1372.
- Ferris, J. P. and D. E. Nicodem. 1972. Ammonia Photolysis and the Role of Ammonia in Chemical Evolution. Nature. 238: 268-269.
- Bada, J. L. and S. L. Miller. 1968. Ammonium Ion Concentration in the Primitive Ocean. Science. 159 (3813): 423-425.
- Bada, J. L. and A. Lazcano. 2003. Prebiotic Soup—Revisiting the Miller Experiment. Science. 300 (5620): 745-756.
- Deamer, D. 2011. First Life: Discovering the Connections between Stars, Cells, and How Life Began. London: University of California Press, Ltd., 25.
- Miller, S. L. 1953. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science. 117 (3046): 528-529.
- Johnson, A. P. et al. 2008. The Miller Volcanic Spark Discharge Experiment. Science. 322 (5900): 404.
- Fox, S. W. and K. Harada. 1958. Thermal Copolymerization of Amino Acids to a Product Resembling Protein. Science. 128 (3333): 1214.
- Leman, L., L. Orgel, and M. R. Ghadiri. 2004. Carbonyl Sulfide-Mediated Prebiotic Formation of Peptides. Science. 306 (5694): 283-286.
- Lehman, N. 2015. The RNA World: 4,000,000,050 Years Old. Life. 5 (4): 1583-1586.
- Gilbert, W. 1986. Origin of life: The RNA world. Nature. 319 (6055): 618.
- Jia, T. Z. et al. 2016. Oligoarginine peptides slow strand annealing and assist non-enzymatic RNA replication. Nature Chemistry. 8 (10): 915-921.
- Stern, V. “Definitely embarrassing”: Nobel Laureate retracts non-reproducible paper in Nature journal. Retraction Watch. Posted on retractionwatch.com December 5, 2017, accessed January 10, 2018.
* Dr. Jeffrey Tomkins is Director of Life Sciences at the Institute for Creation Research and earned his Ph.D. in genetics from Clemson University.